Aug 3, 2022
Recall of Sodium Chloride Injection 23.4% W/V
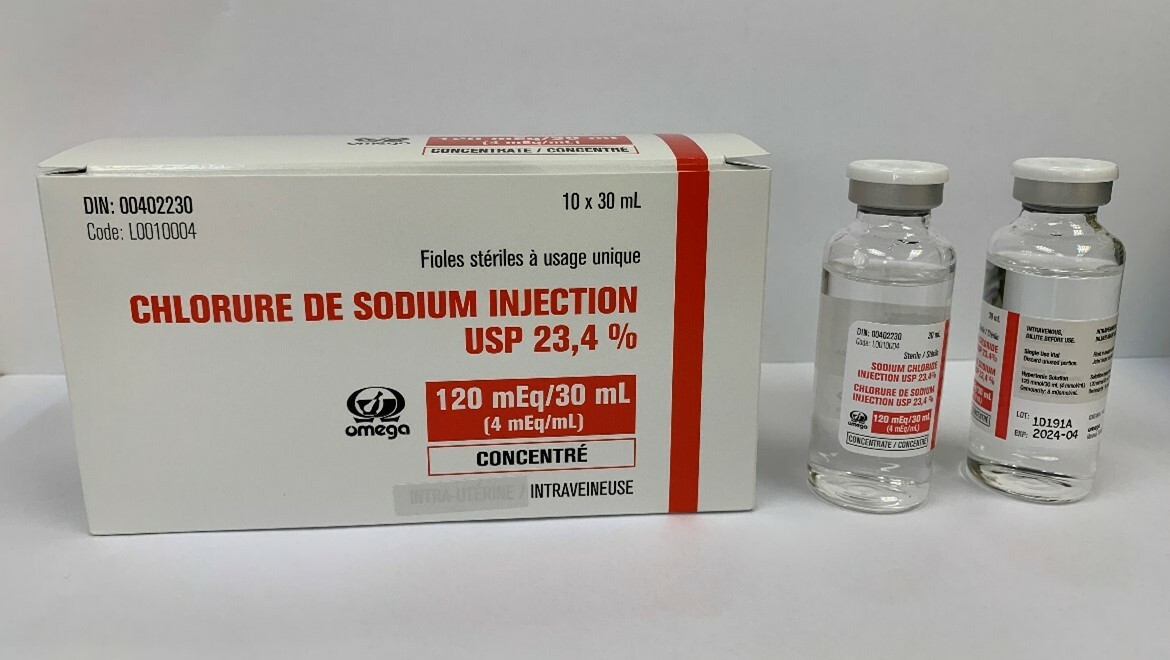
(Hong Kong, 3 August 2022) The Department of Health (DH) today (August 3) endorsed Sino-Asia Pharmaceutical Supplies Limited (Sino-Asia) to recall a total of five batches of Sodium Chloride Injection USP 23.4% (batch no.: 0J970A, 1A106A, 1B134A, 1B142A & 1D191A) from the market because of a potential quality issue.
Health Canada announced that Omega Laboratories Ltd is recalling Sodium Chloride Injection USP 23.4%, 30mL vials following test for glass delamination at the 12 month stability timepoint and the results showed the presence of glass particles.
The above affected batches of the products have been imported into Hong Kong and supplied to Hospital Authority, Private Hospitals and one private doctor.
Sino-Asia has set up a dedicated Recall hotline (852 - 2573 1400) to handle related enquiries.
Sino-Asia will closely monitor the recall and report to DH. So far, Sino-Asia has not received any adverse reaction reports in connection with the impacted batches.










